Test Cases Improve GMP Risk Processes
The operational and performance validation scripts, developed specifically for the Infor Cloudsuite solution, help you reduce the effort, and lessens the risk of errors by facilitating compliance with these stringent requirements. Copley currently provides sixteen different tests cases for various GMP Risk areas.
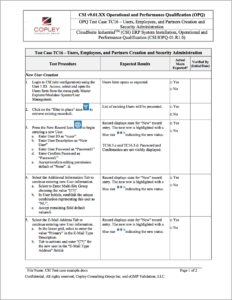
Sample OPQ Test
FDA Validation Tools
Copley’s FDA Extended ERP solution ensures validation of the software’s intended use while exposing a company to their risk of FDA audit non-compliance if not properly executed. Our experience with FDA requirements and manufacturing best practices for clients has led to our development of these templates and an implementation methodology to ensure FDA compliance.

Process Templates
If your Regulatory personnel have experience in leading an FDA Validation project that included the back-end ERP software, then you can leverage your internal resources by having them take the lead in the process. To support their efforts and to maximize their efficiency, the Copley FDA Validation Templates provide guidance to streamline their workload.
Support & Services
Implementing an ERP solution for organizations that fall under FDA regulations must go through a validation process. The validation determines, defines, governs and documents the formal testing of Good Manufacturing Practices (GMP) and relevant activities pertaining to the definition, production and delivery of regulated products and services.
CONTACT US TO
